QLS-215
Our lead program, QLS-215, is designed to treat Hereditary Angioedema (HAE), a rare genetic disorder characterized by recurrent, unpredictable, and potentially life-threatening edema in the skin, abdomen, and airway.
Our vision for the lead program, QLS-215, is to develop the best-in-class monoclonal antibody inhibitor of plasma kallikrein for HAE with infrequent dosing and sustained blood levels. QLS-215 is a humanized monoclonal antibody targeting plasma kallikrein that has demonstrated high potency for plasma kallikrein in an in vitro functional assay as well as extended plasma half-life in non-human primates. We expect to file an Investigational New Drug application for QLS-215 in the first half of 2022 and plan to initiate a Phase 1 clinical trial with initial results anticipated by the end of 2022. Subsequently, we expect to initiate a Phase 1b/2 trial in patients affected by HAE in 2023.
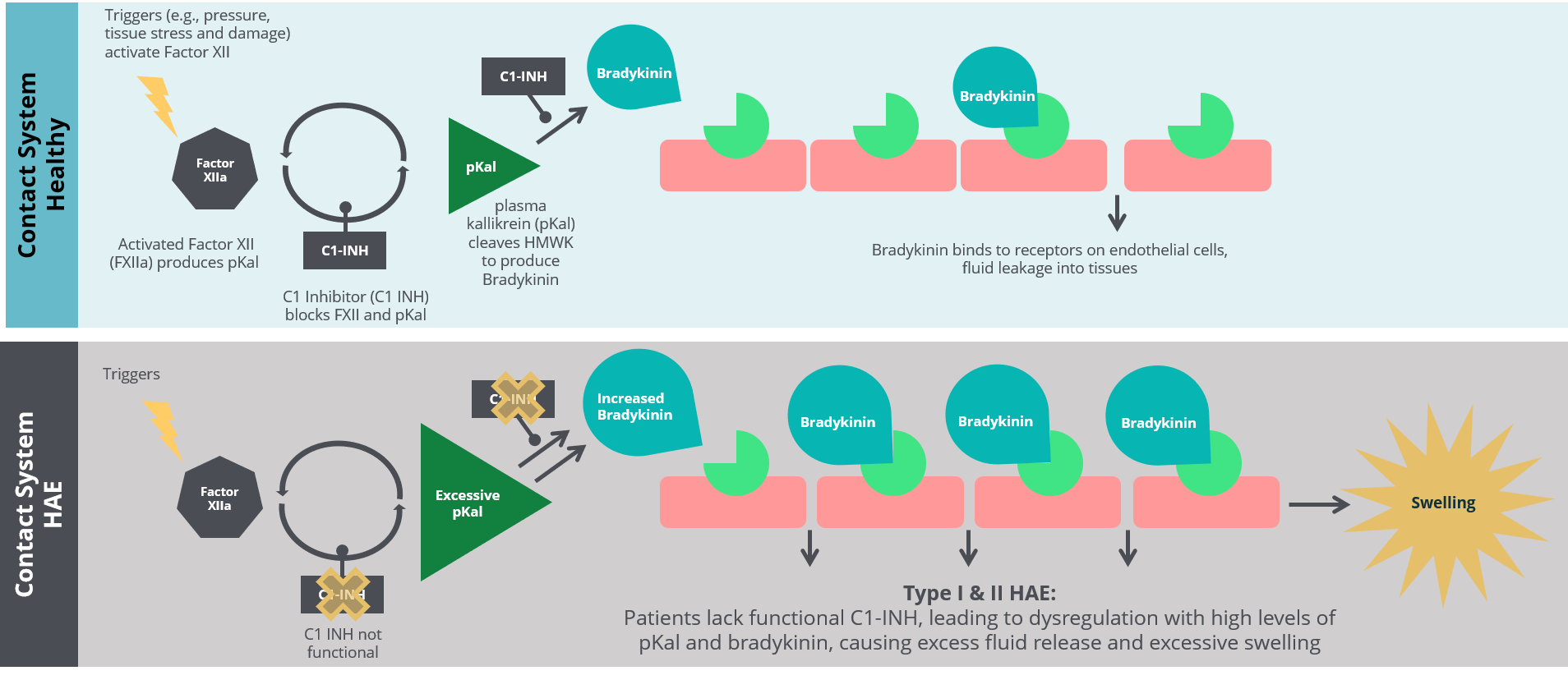
Dysregulation of the plasma contact system mediates excessive swelling underlying HAE disease symptoms
© Copyright 2021 - All Rights Reserved | Terms of Use | Privacy Statement